Eczema: New drug reduces symptoms by 75% in infants and young children
Eczema: New drug reduces symptoms by 75% in infants and young children
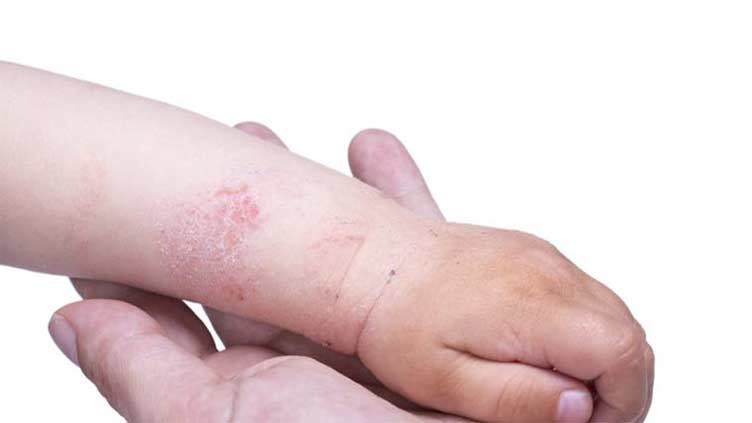
ISLAMABAD, (Online) - Between 15% and 20% of children around the world have eczema — an inflammatory skin condition that is currently incurable.
Because of its symptoms, past research shows children with eczema — clinically known as atopic dermatitisTrusted Source — face higher rates of mental healthTrusted Source issues such as depressionTrusted Source and anxiety. They may also struggle with sleeping.
Now, a phase 3 clinical trial recently publishedTrusted Source in The Lancet reports that the drug dupilumabTrusted Source provided at least a 75% improvement in eczema symptoms by week 16 in children ages 6 months to 5 years old when used in conjunction with a topical corticosteroid.
What is eczema?
Atopic dermatitis is a chronic inflammatory skin disease caused by a variety of reasons, including a person’s inflammatory response, certain triggers, genetic history, and stress.
Symptoms of eczema include:
• extremely dry, flakey, and/or cracked skin
• rashes on the neck, wrists, and ankles, or behind the elbows and knees, in children
• itching
• red, irritated skin
• blisters/sores that easily break and leak fluid
There is currently no cure for atopic dermatitis, but doctors treat flare-ups of the disease as well as provide preventative measures for future outbreaks.
Traditional eczema symptom medications include:
• corticosteroid creams
• immunosuppressant medications
• antihistamines
• antibiotics
Atopic dermatitis in children
Eczema is common in children. Research shows that 60% of childrenTrusted Source first develop eczema symptoms during the first year of life, with some children outgrowing eczemaTrusted Source as they near adulthood.
“Babies and young children with uncontrolled atopic dermatitis may be constantly scratching, irritable, and awake multiple times in the night with their parents, which can impact (the) quality of life for both the children and their parents,” explained Dr. Amy S. Paller, the chair of dermatology and professor of pediatrics at Northwestern University Feinberg School of Medicine in Illinois and a principal investigator of the phase 3 clinical trial.
According to Paller, there was a need for treatment options for infants and young children with uncontrolled moderate-to-severe atopic dermatitis before the Food and Drug Administration approved the use of Dupixent in children ages 6 months to 5 years of age with moderate-to-severe atopic dermatitis in June 2022.
“The treatment options for patients between the ages of 6 months and 5 years were mostly limited to topical steroids,” she explained to Medical News Today. “Given that the options otherwise were only immune suppressants like oral steroids, cyclosporine, and methotrexate, we as pediatric dermatologists were very hesitant to move to anything beyond topical steroids. And, frankly, even worried about using potent steroids chronically, which some of these children with moderate-to-severe eczema need for even partial control.”
What is dupilumab?
Dupixent (dupilumab) is a medication created by Regeneron Pharmaceuticals Inc. and jointly developed by Regeneron and Sanofi.
The Food & Drug Administration (FDA) first approvedTrusted Source Dupixent for the treatment of atopic dermatitis in adults in March 2017.
The FDA then expanded the use of Dupixent in 2020 for children ages 6 to 11 years old before the approval earlier this year for children ages 6 months to 5 years of age.
Paller stated that dupilumab is a biologic medicineTrusted Source that works to help address an underlying source of inflammation — called type 2 inflammation — that contributes to atopic dermatitis. She added that dupilumab is not an immunosuppressantTrusted Source or steroid.
“Many parents and caregivers of babies and young children with uncontrolled moderate-to-severe atopic dermatitis have tried multiple topical therapies, and some have even tried systemic steroids or broad immunosuppressants, and still remain uncontrolled, which leaves them searching for new options,” she explained. “Long-term use of corticosteroids and off-label immunosuppressants can be temporarily effective but can also be associated with significant side effects.”

