Doctors treat first U.S. coronavirus patients with convalescent plasma therapy
More than 454,000 people in the U.S. have tested positive for COVID-19
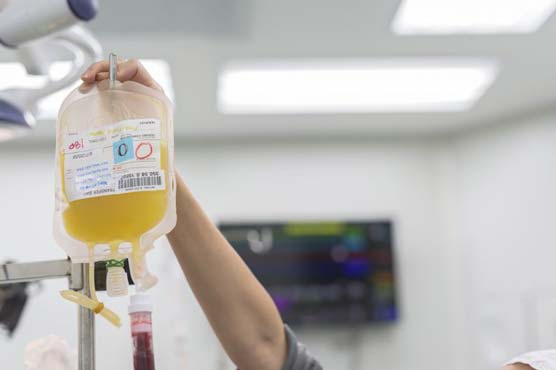
U.S. hospitals desperate to help very sick patients with COVID-19, the highly contagious respiratory disease caused by the new coronavirus, are trying a treatment first used in the 1890s that relies on blood plasma donated by recovered patients.
People who survive an infectious disease like COVID-19 are generally left with blood containing antibodies, or proteins made by the body’s immune system to fight off a virus. The blood component that carries the antibodies can be collected and given to newly infected patients - it is known as "convalescent plasma."
More than 454,000 people in the U.S. have tested positive for COVID-19, more than 16,000 have died as of Thursday (April 9), according to Johns Hopkins University.
Convalescent plasma treatment is not new. It was successfully used during the 1918 flu pandemic.
"Plasma has a good track record in the past, but coronavirus is a new disease," said Johns Hopkins Bloomberg School of Public Health Chair of Molecular Microbiology and Immunology Dr. Arturo Casadevall.
"We’re going to have to learn how to use it. And even though it is encouraging, and even some of the early reports are positive, I think I think that we need to be rigorous in our thinking and to test this appropriately with clinical trials."
Casadevall is one of the doctors leading the National COVID-19 Convalescent Plasma Project.
"This is going to be something that will hopefully help us stem the epidemic, but by no means is a panacea," he said.
"We’re going to need a lot of things to conquer coronavirus, including vaccines, drugs, better antibodies, a lot of things in the future. This is just one of the many options that could help us."
On March 28, two hospitals in the U.S. began treating COVID-19 patients with convalescent plasma; Houston Methodist and Mount Sinai Medical Center in New York City.
Dr. Ania Wajnberg (pronounced Anya Wineberg) directs Mount Sinai’s Serum Antibody Program.
"About 35 patients have received plasma at Mount Sinai. The first one was not even two weeks ago, about a week and a half ago. So we don’t yet have enough data to say our findings, but we are hopeful that this is going to be helpful for these patients. We are tracking them incredibly carefully for their clinical progress, and other data that we use to monitor and hopefully in about two weeks we will be able to tell you and the world what we’re finding so far."
The convalescent plasma therapy process takes up to 90 minutes, and plasma from a single donor can be used to treat three or four patients.
Donors must have been diagnosed with COVID-19 and need to wait a defined period of time after they test negative for the disease before donating plasma. Tests are also being developed to measure antibody volume.
Wajnberg said the trials will seek to answer several questions: "Can they be reinfected or not? How long will they remain immune? How long will they remain at a high level versus a low level? Those are all things that we plan to look at, and have huge implications on our healthcare workforce and potentially the workforce of the world as we reopen society."
In one trial in China, levels of the virus in five seriously ill COVID-19 patients were undetectable after plasma transfusions, according to study results published in late March in The Journal of the American Medical Association.
"I think we’re going to learn more and more as we go," said Wajnberg. "We just need to monitor them for a couple of weeks and see how they do."
"What I will tell you so far is that we haven’t seen any bad side effects, which is also really important when you’re starting a new treatment. So that’s encouraging and I hope in like the next two weeks, we’ll have more data to share," she said.
"Ultimately our goal is to see them recover."

